Rudolf Sillén has written an article on Bubbles, sugar & sweetness in Champagne. [read the full champagne story]
Estimated reading time: 4 minutes

A second fermentation in the bottle is the origin of “bubbles”.
The most distinctive thing about Champagne and sparkling wines is that it “bubbles” when poured into a glass. The bubbles consist of carbon dioxide (CO2), which is a colorless and odorless gas that is about 50 % heavier than air. Density 1.98 g/dm3. The carbon dioxide has been formed by adding sugar and yeast to a still, white wine so that a second fermentation can take place in the bottle. The normal addition is about 24 grams of sugar per liter and a little yeast. It is called “Liqueur de tirage”. This second fermentation produces approx. 1.2 % alcohol and about 12 liters of carbon dioxide, which creates a pressure of about 6 bar in the bottle. Carbon dioxide dissolves in the wine and carbonic acid is formed via a reversible reaction (CO2+H2O <-> H2CO3).

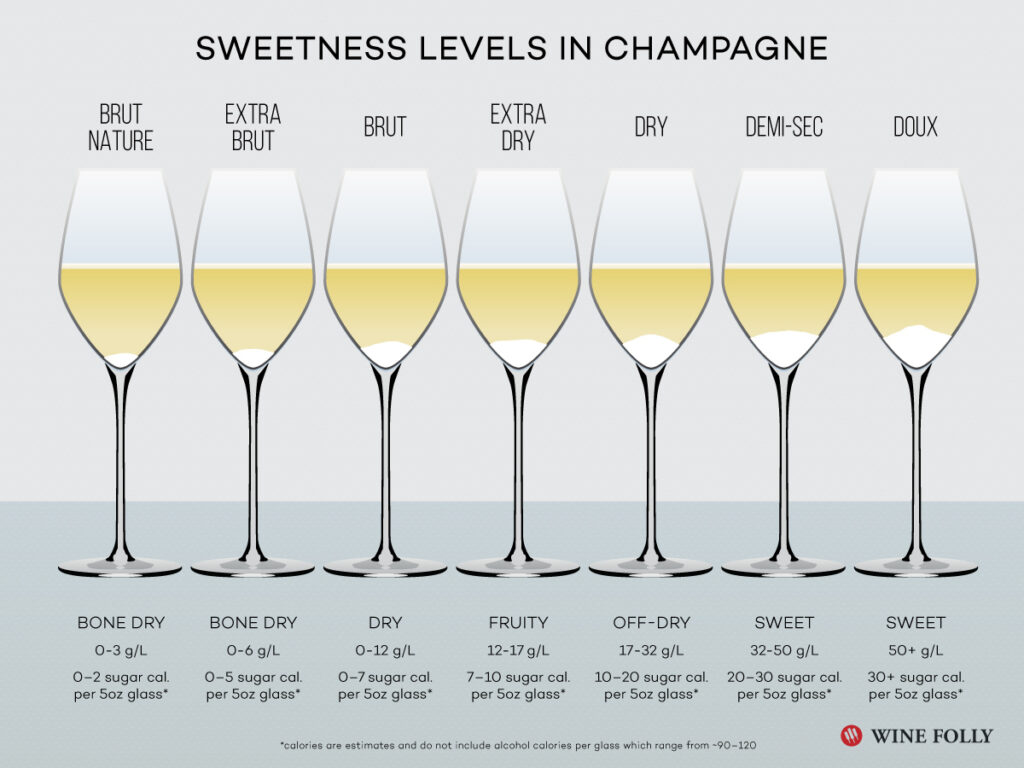
Some of the carbonic acid forms hydrogen and carbonate ions (H+ and HCO3-), which makes it a weak acid. After this second fermentation, the sugar content is usually less than 3 grams per liter and the Champagne is then classified as “Brut nature”. At this stage the champagne has a low pH (often <3) and a high total acid (acidity, often >7 g/l)). The high level of carbon dioxide in the wine makes it feel fresh, “spritzy” in the mouth. Enzymes in the mouth convert carbon dioxide into carbonic acid, which the taste buds perceive as slightly sour (without added sugar). Indications are that aromas are felt more clearly at high levels of carbon dioxide. (My hypothesis: CO2 molecules act as “carriers” of aroma molecules?)
Added sugar and acid balance the sweetness.
Sugar is often added to the wine after the second fermentation to balance the acidity. The addition is called “Liqueur d´expedition” and is added after the wine has been disgorged and the dead yeast removed. Sweetness depends not only on sugar but also on alcohol, glycerin and acidity. High acidity reduces sweetness. The carbon dioxide and acidity are significantly higher in sparkling than in most still wines. Therefore rather high levels of sugar are needed for the wine to be perceived as sweet. Up to the “Brut” classification, which allows up to 12 grams of sugar per liter, Champagne is usually experienced as quite dry because the carbonation overshadows the sugar. Only at the “Sec / dry” classification, which means 17-32 grams/liter, does a certain sweetness begin to be perceived.

The champagne transforms into a still wine and makes the sweetness obvious.
When you pour Champagne into a glass, the pressure drops from the bottle pressure of about 6 bar (3 times higher than in a car tire) to the atmospheric pressure of 1 bar, which causes the solubility of carbon dioxide to decrease drastically. That causes the carbon dioxide to bubble up and disappear. The reduction of carbon dioxide causes the Champagne to gradually change. The pH increases slightly at the same time as carbonation and acidity decrease. After about 30 minutes, the “bubbling” has come to an end and the Champagne has turned into a still, sweet white wine with maybe 30 grams of sugar per liter. Without bubbles, the wine now feels very sweet and unbalanced.
In a test with a Sec Champagne (30 g sugar/liter) we let a group of tasters estimate sweetness on a scale from 1-5. After filling the glasses the average experience of sweetness was 2. We waited 30 minutes until no more bubbles could be seen. Now the sweetness had increased and was experienced as 4 on the 5 point scale.
Conclusion:
To maximize the enjoyment of Champagne and other sparkling wines, they should be drunk shortly after filling the glass. Preferably within 10 minutes!
Referenses:
- How does dissolved carbon dioxide affect taste and texture of still white and red wine? Dr. Richard Gawel. Australien Wine Research Institute
- The role of CO2 in wine production. Atlas-Scientific.com
- Understanding Wine Technology. Dr. David Bird. Chemist.
- Mail conversation.
- Wine Tasting. A Professional Handbook. Prof. Ronald S. Jackson.
- Plus numerous metastudies via Google and YouTube. Mail conversation with Systembolaget Lab.
- Mail conversation with Richard Juhlin.